PROTECT Participant Information Animation
The PROTECT Study is comparing two types of glucose monitoring for pregnant women with Type 2 diabetes.
All pregnant women with Type 2 diabetes are asked to monitor their glucose levels every day.
This is to make sure that babies don’t get too much glucose and become too big as this can cause problems during and after birth, for both mother and baby.
We don’t know which type of glucose monitoring works best.
The two types we’re comparing are finger-prick monitoring and Continuous Glucose Monitoring or CGM.
Finger-prick monitoring is the most common.
The mother pricks her finger with a small device at least 4 times a day.
The drop of blood is placed onto a strip that’s put into a glucose reader.
The glucose level is recorded in a diary and shared with the clinical team to decide what treatment is needed.
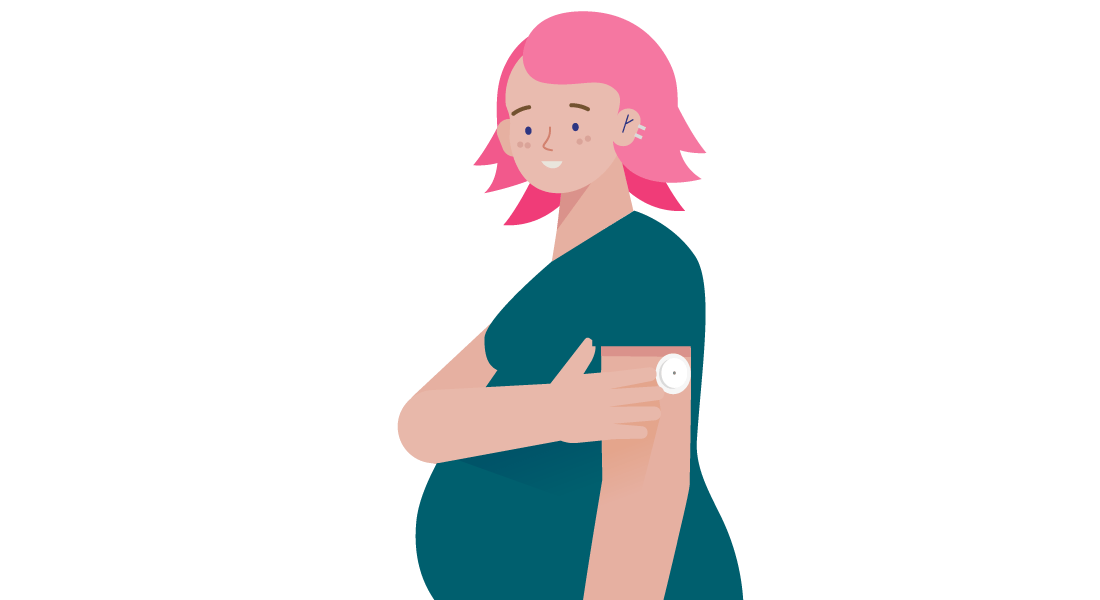
A newer way of monitoring glucose levels is CGM.
The mother wears a small sensor for two weeks at a time.
The sensor measures glucose levels every minute and sends them to an App on the mother’s phone.
This is shared with the clinical team to decide what treatment is needed.
Both options are used across the NHS so there are no additional risks to you or your baby.
You and your baby will be looked after throughout your pregnancy whichever type of monitoring you use.
You will only be invited to take part if your diabetes pregnancy team thinks you are suitable.
If you agree to join the study we will ask you to sign a consent form and we will collect some general health information.
For the first 1-2 weeks you will do finger-prick monitoring and wear a ‘masked’ sensor, which hides the glucose data from the wearer. This is to get your starting glucose levels and check you are OK wearing the sensors.
After 1-2 weeks you will be randomly allocated to either finger-prick or CGM for the rest of your pregnancy.
Neither you nor your doctor can choose which you receive.
If you are allocated to finger-prick monitoring we will ask you to wear a masked CGM on four other occasions.
During the study we will record information about you and your baby's health.
This will be stored confidentially and securely.
Once your baby is born there will be no more study visits.
Joining the study is voluntary and you can leave at any time.
If you want more information please speak to your diabetes pregnancy team.